Sedimentary rocks
Sedimentary rocks
Introduction
All rocks on the surface of the Earth are exposed to the effects of erosion. The products of erosion accumulate to form sediments. Young sediments are unconsolidated (loosely connected) but with time they become consolidated and form sedimentary rocks. Sediments form on the surface of the Earth (most often underwater) and occur in layers – with the youngest layers at the top.
The thickness of sediments on the surface of the Earth varies widely, from totally absent (for example at mid-ocean ridges) up to a maximum of ca. 20 km. Sedimentary rocks cover ca. 80% of the surface of the Earth, but contribute less than 1% to its mass. Sediments are, however, economically very important because they contain the major energy sources – coal, oil, and gas.
6.2 Weathering
There are two types of processes that cause the alteration of rocks exposed on the surface of the Earth – physical and chemical. Physical or mechanical alteration results in rocks breaking into smaller fragments; these rock fragments are called detritus. Different grain sizes of detrital fragments are named as shown in Table 6.1.
Term
Size range (mm)
boulder >256
cobble 64–256
pebble 2–64
sand 1/16–2
silt 1/256–1/16
mud < 1/256
Grain size of unconsolidated sediments
Boulders, cobbles and pebbles are coarse-grained, sand is medium-grained, and silt and clay are fine-
grained.
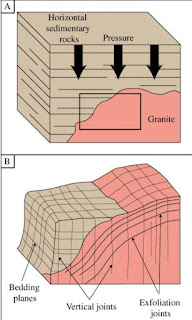
There are a variety of processes that result in physical weathering. Rocks buried in the crust are under pressure because of the weight of the overlying deposits and hotter than at the surface (the increase in temperature with depth is usually in the range 20–40°C/km). Joints form when pressure is reduced as a result of uplift and erosion. The type of joints depends on the kind of rock involved
Loose blocks of rock resulting from jointing can accumulate at the base of rock exposures to form talus. There are several agents that can cause joints to open and rocks to form fragments. These include frost-wedging (water enters cracks and expands on freezing; most effective where freezing and thawing alternate rapidly) (Fig.6.2), root-wedging, salt-wedging, thermal expansion and animal influence (including humans).
Introduction
All rocks on the surface of the Earth are exposed to the effects of erosion. The products of erosion accumulate to form sediments. Young sediments are unconsolidated (loosely connected) but with time they become consolidated and form sedimentary rocks. Sediments form on the surface of the Earth (most often underwater) and occur in layers – with the youngest layers at the top.
The thickness of sediments on the surface of the Earth varies widely, from totally absent (for example at mid-ocean ridges) up to a maximum of ca. 20 km. Sedimentary rocks cover ca. 80% of the surface of the Earth, but contribute less than 1% to its mass. Sediments are, however, economically very important because they contain the major energy sources – coal, oil, and gas.
6.2 Weathering
There are two types of processes that cause the alteration of rocks exposed on the surface of the Earth – physical and chemical. Physical or mechanical alteration results in rocks breaking into smaller fragments; these rock fragments are called detritus. Different grain sizes of detrital fragments are named as shown in Table 6.1.
Term
Size range (mm)
boulder >256
cobble 64–256
pebble 2–64
sand 1/16–2
silt 1/256–1/16
mud < 1/256
Grain size of unconsolidated sediments
Boulders, cobbles and pebbles are coarse-grained, sand is medium-grained, and silt and clay are fine-
grained.
There are a variety of processes that result in physical weathering. Rocks buried in the crust are under pressure because of the weight of the overlying deposits and hotter than at the surface (the increase in temperature with depth is usually in the range 20–40°C/km). Joints form when pressure is reduced as a result of uplift and erosion. The type of joints depends on the kind of rock involved
Fig.6.1: Development of joints in granites and sediments as a result of uplift and erosion. Granites (homogeneous, massive rocks) commonly form onion-like sheets, whereas sediments develop vertical fractures.
Fig.6.2: Cracks opening as a result of frost wedging. This is a very efficient way of fracturing rocks.
Rocks are also affected by chemical weathering. Water plays a key role here because of chemical reactions with rock-forming minerals. Chemical weathering is most effective in warm climates. Halite (rock salt, NaCl) dissolves in pure rainwater, whereas calcite (CaCO3) is only soluble in acidic water. Rainwater dissolves CO2 from the air and forms carbonic acid H2CO3. This can react with calcite:
CaCO + H CO = Ca2+ + 2(HCO )- 323 3
Because of the solubility of calcite, the rocks limestone and marble (metamorphosed limestone) dissolve in wet climates. Joints become wider and underground rivers and caves can form.
Water can react with other minerals. K-feldspar (important in e.g. granite) reacts with slightly acidic water to form kaolinite (a clay mineral).
2K[AlSi3O8] K-feldspar
+ 2H2CO3 + H2O = carbonic acid water
Al [Si O ](OH)
kaolinite potassium bicarbonate silica in (clay mineral) ions ions solution
Many other silicate minerals also react slowly with (acidic) water to form clay minerals (a process called hydrolysis). These include olivine, pyroxenes, amphiboles and micas (all dark minerals). Quartz, however, is chemically resistant and is often the only mineral to survive extensive chemical weathering.
Oxidation can play an important role in the chemical alteration of minerals. Iron is the critical element in this context because it exists in two oxidation states, Fe2+ and Fe3+. The oxidation (rusting) of iron is a well-known phenomenon, not least to car owners. Two reactions are involved:
4Fe2+ + 3O2 = 2Fe3+2O3 ferrous iron hematite
4Fe2+ + O2 + 2H2O = 4Fe3+O(OH) goethite
Both the minerals hematite and goethite are components of rust.
Physical and chemical weathering operate together. Mechanical processes give more cracks (fractures) and thereby a greater surface area. Chemical processes, which operate on surfaces, are therefore enhanced by extensive fracturing (Fig.6.3). Minerals become altered at different rates. Quartz is a very resistant mineral and is often the only one to survive chemical weathering; all the others alter to clay (Fig.6.4). Sandy beaches are usually dominated by quartz.
Fig.6.3: The efficiency of chemical weathering increases as the surface area increases because of mechanical weathering.
The two weathering processes work together.
Fig.6.4: Minerals alter at different rates.
Feldspar and dark minerals in granite alter relatively rapidly to clay minerals whereas quartz is very resistant. The clay fraction is easily removed, leaving quartz sand as the final product of weathering.
Both mechanical and chemical alteration proceed faster on edges – and faster still on corners. This commonly results in rectangular blocks becoming rounded – so-called spherical weathering (Fig.6.5).
Fig.6.5: Extensive weathering results in the development of rounded boulders. This is a consequence of weathering being a surface effect.
Classification of sedimentary rocks
Weathering processes form rock fragments, grains of resistant minerals, new minerals (e.g. kaolinite, a clay mineral) and ions in solution. Particles can be transported by wind, water or ice until they are deposited. Dissolved ions can enter the groundwater and/or be transported by streams and rivers to the sea. These ions form new minerals in the sea or in pore spaces underground. Organisms can play an important role.
There are four different types of sedimentary rocks:
• clastic – (or detrital) – consist of consolidated fragments
• biochemical – consist of the shells of organisms
• organic – consist of carbon-rich plant remains
•chemical – consist of minerals deposited directly from hydrous solutions
6.3.1 Clastic sedimentary rocks
Loose detritus becomes a clastic sedimentary rock via a series of events (Fig.6.6).
Fig.6.6: Formation of clastic sedimentary rocks involving weathering, transport and deposition.
An immature sediment (breccia) consists of angular fragments with a variety of grain size (poorly sorted). As a sediment becomes more mature the grains become increasingly rounded, smaller, and more uniform in size. The final product illustrated here is a mature sandstone.
a) Weathering (dominantly physical) forms fragments (detritus).
b) Transport. Rock fragments fall off the outcrop and/or are moved by wind, water or ice. Since
water and wind move small particles further than large ones they become sorted by size during transport. The combined processes of weathering and transport are referred to as erosion.
c) Deposition. Particles settle out of the medium of transport. For example when a glacier melts it deposits its load of rock particles. When flowing water slows down the coarsest particles settle out, or when the wind dies down sand particles are deposited.
d) Lithification (consolidation) is the process whereby loose sediment is transformed into solid rock. The pressure resulting from burial under younger sediments presses water and air
out of pore spaces and results in compaction. Sand can compact by 10–20%, whereas mud (a mixture of clay and water) can compact by as much as 50–80%. Compacted sediment may then be cemented by the deposition of new minerals (usually quartz or calcite) that
are derived from groundwater. Lithification is the combined result of compaction and cementation
(Fig.6.7).
Fig.6.7: The sedimentary rock-forming process (lithification).
This involves a combination of compaction and cementation. The cementing agents are components carried by groundwater.
As rock (and mineral) fragments are transported away from their source they become smaller and change their shape. Sediments deposited near the source will be very different from those deposited far away after considerable transport.
A clastic sedimentary rock that forms very close to its source will contain large, angular fragments. These large fragments (clasts) will occur in a matrix (or groundmass) consisting of smaller particles. This type of rock is a breccia. The fragments can consist of igneous, metamorphic or sedimentary rocks (or a combination of these), depending on the nature of the rock type(s) that have been eroded.
During transport the angular fragments become rounded. A clastic sedimentary rock with large, rounded fragments in a finer grained matrix is called a conglomerate. Like breccias, the nature of the large particles in a conglomerate reflect the nature of the rock types that have been eroded.
With greater transport the fragments become smaller because of collision with each other, and they become exposed to further alteration. Dark minerals and feldspar become hydrolysed and turn into clay minerals that are transported away. The clasts are commonly dominated by quartz grains, and the rock which forms is a sandstone (i.e. particles in the size range 1/16–2 mm). In a river, sand-sized particles may be deposited in bars. If sand-sized particles reach the estuary of a river they will be deposited in the coastal region. A special type of coarse-grained sandstone containing feldspar as well as quartz grains is called arkose.
Smaller particles can be transported further but will be deposited at some stage to form siltstone after lithification (particles in the size range 1/256–1/16 mm). The smallest, clay-sized particles are transported even further and are deposited as mud. After lithification mud becomes mudstone. Clay particles, however, commonly form minute flakes that are orientated parallel with the bedding plane. After compaction (and cementation) these very fine-grained clastic rocks have a well-developed parting and the rock is called a shale.
6.3.2 Biochemical sedimentary rocks
Organisms can play a major role in the formation of sedimentary rocks. Many organisms have shells of CaCO3 (calcite or its polymorph aragonite). Others have shells of silica (SiO2). When these organisms die their shells can accumulate to form a biochemical sediment. The soft part of the organism rots away – or becomes transformed into oil. Plants can also contribute an organic component to sediments, as we will see in section 6.3.3.
The environment around a coral reef is extremely rich in organisms such as corals, algae, oysters, clams and snails. Plankton float in the water. All these have shells of CaCO3 (calcite or aragonite). When the organism dies, its skeleton remains where it is (e.g. coral reef) or is transported away. During transport the skeletal material can break into small fragments. When this material is deposited it forms a calcium carbonate-rich sediment – limestone. Since this is largely composed of the remains of organisms it is a biochemical sediment. A different type of limestone can be precipitated directly from aqueous solutions without the influence of organisms (e.g. stalagmites and stalactites in caves). This type of limestone is of chemical origin and will be dealt with later. Limestones are sometimes formed in an environment where clay is deposited simultaneously. This gives rise to a rock type known as marl – a mixture of limestone and clay.
There are many varieties of limestone of biochemical origin. Some are dominated by coral reefs in their original position (reef limestone). Others consist largely of shells and shell fragments (fossiliferous limestone). Some contain small spheres of calcite (oolites; oolitic limestone) formed by coatings of calcite around small particles (usually shell fragments or quartz grains). These form in agitated, shallow water. Some consist of microscopic shells of plankton called foraminifera (chalk). Lime mud can consolidate to give very fine-grained limestone called micrite.
The shells of some organisms, most notably those of a type of plankton called radiolaria, are composed of silica (SiO2). These tiny shells accumulate on the deep sea floor as a silica-rich ooze. After burial beneath younger deposits these solidify to form a type of cryptocrystalline quartz called chert. These chert deposits only develop in the deep ocean and commonly form bands (banded chert).
6.3.3 Organic sedimentary rocks
Coal is an organic sedimentary rock. It is formed from the remains of plants that grew in swamps or forests. The plant remains became buried and, after being subjected to elevated temperatures and pressures, were converted to the black, combustible rock known as coal which consists of >50% carbon.
The soft parts of plankton can mix with mud on the sea floor and be incorporated into shale. This organic material (which is gradually converted into oil) colours the shale black. Such black shales are called oil shales.
6.3.4 Chemical sedimentary rocks
Chemical sediments form by the precipitation of minerals from aqueous solutions. There are three main types of chemical sediments:
• evaporites – formed by the evaporation of salt water
• travertine – carbonate rocks precipitated from water
• dolomite and chert – formed by the replacement of other rocks
Evaporites. The evaporation of salt water leaves a residue of salt. For salt water to evaporate it requires a closed system (e.g. a salt lake with no outlet) and a warm climate. Salt water contains many other ions in solution than Na+ and Cl-, and a variety of minerals form in a regular sequence during the evaporation of salt water. The first mineral to form is gypsum (CaSO4.2H2O) when about 80% of the water has evaporated, followed by halite (NaCl) when about 90% has evaporated. After this, a sequence of relatively rare evaporite minerals may form (including the potassium equivalent of salt, sylvite KCl).
As we have seen, limestone can form by the accumulation of biochemical material. It can also, however, form by direct precipitation from water without organisms being involved. This chemical variety of limestone is called travertine. Water, especially acidic water, can dissolve calcite in limestone. The carbonate material is, however, commonly precipitated again, often in limestone caves (as stalagmites and stalactites etc.) or around hot springs. Travertine, which is usually banded and beige in colour, is widely used as a facing stone.
Some rock types are formed by the replacement of pre-existing sediments. The question then arises as to whether it is reasonable to call them sedimentary rocks? The processes of burial (and the resulting compaction) and cementation are included in the “sedimentary” realm. Processes that take place after deposition, but not involving particularly elevated temperatures and/or pressure (which result in metamorphism which is dealt with in Chapter 7), are referred to as diagenesis and play a very important role in the formation of solid rocks from loose sediments.
Dolomite (CaMg(CO3)2) is a carbonate mineral in which half the calcium in calcite is replaced by magnesium. Dolomite forms as a result of reaction between calcite and Mg-bearing groundwater. Calcite can become partially replaced by dolomite. This replacement can take place soon or long after formation of the limestone. The term dolomite is used both for the mineral CaMg(CO3)2 and the rock that is formed.
Chert is an extremely fine-grained (cryptocrystalline) variety of quartz. Black chert is called flint. Chert/flint are very fine-grained and almost glassy with a choncoidal fracture. Most plankton have shells composed of carbonate, but some have shells composed of SiO2. This silica, which is distributed throughout biochemical limestone deposited on the sea floor, becomes dissolved by percolating water and may be deposited elsewhere. The deposition of chert/flint usually starts around/on “impurities” present in the limestone – such as larger shell fragments (for example sea urchins). Deposition may continue along bedding planes, form nodules or take place in an irregular fashion. Reddish chert is called jasper. Fossilised wood has usually been replaced by chert, and detailed structures (such as tree rings) may be superbly preserved during this process. Agate is banded chert that has been precipitated in a cavity (usually in lava) and has been deposited inwards from the walls. Many commercial agates have been artificially coloured.









Comments
Post a Comment